The chart below shows the calculated isotope pattern for the formula Na 2 O 2 with the most intense ion set to 100% ReferencesSodium peroxide has the formula Na₂O₂ What is its empirical formula?SODIUM CARBONATE PEROXIDE can be found in 232 products Print Share on Evidence Health issue Level of Concern Source Component HYDROGEN PEROXIDE ATSDR states that inhalation of household strength hydrogen peroxide (3%) can cause respiratory irritation Inhalation of vapors from concentrated (over 10%) hydrogen peroxide can cause

Answered Write The Correct Chemical Formula For Bartleby
Sodium peroxide added with water formula
Sodium peroxide added with water formula-An empirical formula is a chemical formula that tells us the relative number of atoms for each element in a compound, and we express it in the simplest wholenumber ratiosWrite the chemical formula of the following compounds Sodium periodate Hydrogen peroxide ammonium carbonate thioacetamide 12 Points) Enter your answer 7 Complete the following equations 12 Points) 1 BaC, 0))K;Croa) 2 CO POCHO
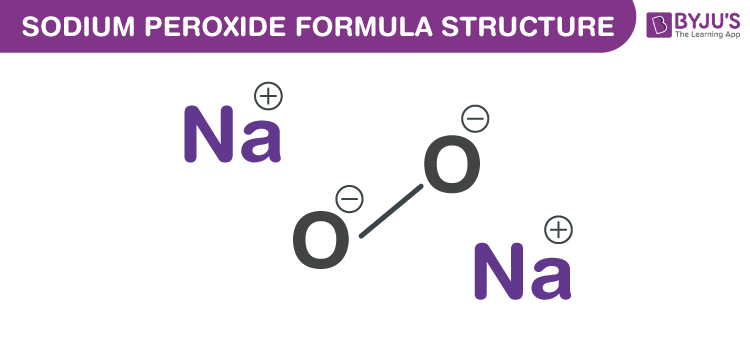


Sodium Peroxide Formula Chemical Formula Structure And Properties
Premium Sodium Percarbonate 999% Pure Anhydrous 2 Pounds (Solid Hydrogen Peroxide Oxygenated Bleach) MultiUse Safe in Home Ecoxall Chemicals 45 out of 5Formula Weight 0 100(5) 101 0(4) 1 300(4)Sodium Carbonate Peroxide is the inorganic salt that conforms to the formula 2Na 2 CO 3 • 3H 2 O 2
Sodium peroxide none listed none listed none listed Sodium hydroxide 2 mg/m3 Ceiling 10 mg/m3 IDLH 2 mg/m3 TWA OSHA Vacated PELs Sodium peroxide No OSHA Vacated PELs are listed for this chemical Sodium hydroxide No OSHA Vacated PELs are listed for this chemical Molecular FormulaNa2O2 Molecular Weight7797 Section 10 Stability andThe intended mixture was 40 g sodium peroxide, 02 g dextrose, and 02 g potassium nitrate;Jul 15, 16 · What is the formula for sodium peroxide?
Find here Sodium Peroxide, Disodium peroxide manufacturers, suppliers & exporters in India Get contact details & address of companies manufacturing and supplying Sodium Peroxide, Disodium peroxide, Na2O2 across IndiaActual proportions were 035 g, 259 g, and 02 g respectively There was insufficient sodium peroxide to dissolve decomposition gases, hence a rapid temp and pressure buildup caused the Parr bomb to burstSodium Hydrogen Carbonate formula, also known as Sodium Bicarbonate, formula or Baking Soda formula is explained in this article It is an inorganic compound which is weakly basic and is composed of cations (Sodium) and anions (Bicarbonate) The chemical or molecular formula of Sodium Hydrogen Carbonate is NaHCO 3


Sodium Peroxide Na2o2 Chemspider



Sodium Hydroxide Solution 32 Weight Ar Chem Lab Home Fisher Scientific
List of Peroxide Compounds, Common Compounds of Peroxide O2, Formula, Molecular WeightFormula Weight 101 0 (1) 401 500 (1) 501 600 (2) Melting Point (°C) 0 100 (1) 101 0 (1) Feature Greener Alternative (1) Application sodium peroxide sulfate Advanced Search Structure Search Phenylphosphonic acid 1 Product ResultStructure, properties, spectra, suppliers and links for Sodium peroxide,


Sodium Peroxide Wikipedia



Sodium Peroxide 96 Acros Organics 25g Sodium Peroxide 96 Acros Organics Fisher Scientific
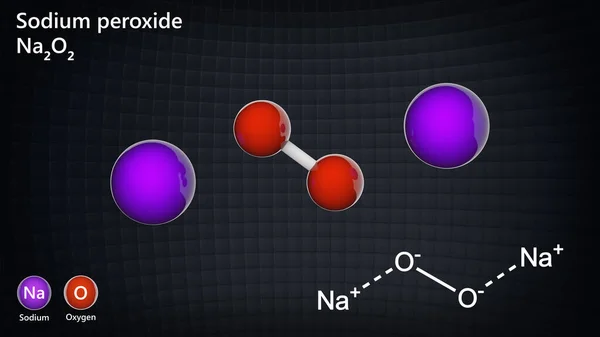
Chemistry Q&A Library Oxidation of the chromium 3 ion (Cr* aq) by sodium peroxide (Na2) in alkaline solution produces sodium chromate (Na2CrO4) and hydroxide ions Write balanced half equations for this reaction, and hence produce a balanced overall equation Show all workingSodium peroxide is the inorganic compound with the formula Na₂O₂ This yellowish solid is the product of sodium ignited in excess oxygen It is a strong base This metal peroxide exists in several hydrates and peroxyhydrates including Na₂O₂·2H₂O₂·4H₂O, Na₂O₂·2H₂O, Na₂O₂·2H₂O₂, andSodium Peroxide Na2O2 Molar Mass, Molecular Weight • Na2O2 H2SO4 = Na2SO4 H2O2



Epa1 Polymeric Diacyl Peroxides Google Patents



Sodium Peroxide Formula Chemical Formula Structure And Properties
MSLRPWGRFCKNIZUHFFFAOYSAJ Sodium carbonate peroxide Similar structures search, synonyms, formulas, resource links, and other chemical informationFeb 06, · Sodium oxide can be prepared via several routes The most common method involves burning sodium in air The reaction produces both sodium oxide and sodium peroxide, with the latter being around % of the product The reaction of metallic sodium with sodium hydroxide, sodium peroxide or sodium nitrite will also give sodium oxide51 Sodium carbonate peroxyhydrate is the chemical name for an addition product produced by drying hydrogen 52 peroxide in the presence of sodium carbonate (CAS No ) The pure substance contains 325 % hydrogen 53 peroxide and 675 % sodium carbonate (based on weight) It combines dual properties of sodium carbonate and


Is Naoh An Acid Or A Base Quora



Molecular Formula Of Sodium Peroxide Water Silver Nitrate Potassium Carbonate Sodium Carbonate Zinc Brainly In
This WebElements periodic table page contains sodium peroxide for the element sodium Li Be Na Mg K Ca;Sodium peroxide, granular for analysis Sodium peroxide replaces and is the same as SX Part # Change only, Same Pack Size, Same Specifications FormulaADR/RID SODIUM PEROXIDE IMDG SODIUM PEROXIDE IATA Sodium peroxide Passenger Aircraft Not permitted for transport 143 Transport hazard class(es) 144 ADR/RID 51 Packaging group IMDG 51 IATA 51 ADR/RID I IMDG I IATA I 145 Environmentalhazards ADR/RID no IMDG Marine pollutant no IATA no 146 Special precautions for user No data



Sodium Hydroxide Wikipedia



What Is Sodium Hydroxide Formula Reactions Video Lesson Transcript Study Com
Chemistry Ionic Bonds Writing Ionic Formulas 1 Answer anor277 Jul 15, 16 #Na_2O_2# Explanation Oxygen in peroxide dianion has a formal #I# oxidation state Is this consistent with the given charge of the ion?Formula Na2O2 Derivation Sodium Peroxide is obtained by adding the peroxide of hydrogen to an excess of caustic soda solution of twenty per cent, and then pouring into alcoholChemically considered, it is the analogue of peroxide of hydrogen PropertiesSodium peroxide definition, a yellowishwhite, hygroscopic, watersoluble powder, Na2O2, used chiefly as a bleaching agent and as an oxidizing agent See more


Cobalt


Structural Chemical Formula And Molecular Structure Of Hydrogen Peroxide H2o2 Chemical Structure Model Ball And Stick 3d Illustration Larastock
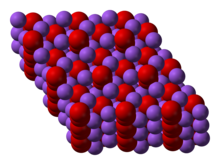
Sodium Peroxide is a highly insoluble thermally stable Sodium source suitable for glass, optic and ceramic applications Oxide compounds are not conductive to electricity However,certain perovskite structured oxides are electronically conductive finding application in the cathode of solid oxide fuel cells and oxygen generation systemsSodium peroxide is the inorganic compound with the formula Na2O2 This yellowish solid is the product of sodium ignited in excess oxygen It is a strong baseSodium peroxide crystallizes with hexagonal symmetry Sodium peroxide was used to bleachShop Sodium Peroxide (Certified ACS), Fisher Chemical at Fisherscicom



Gef 9z5fxtempm
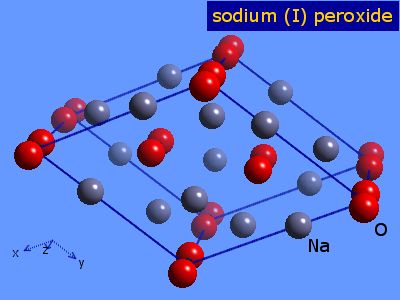


Webelements Periodic Table Sodium Sodium Peroxide
Sodium;peroxide NaO2 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safetyExamples are sodium peroxide (Na 2 O 2), a bleaching agent, and barium peroxide (BaO 2), formerly used as a source of hydrogen peroxideSodium peroxide (Na2O2) hydrolyzes to give sodium hydroxide (NaOH) and hydrogen peroxide(H2O2) when it is treated with water according to the reaction Na2O2 2H2O > 2NaOH H2O2 The reaction is highly exothermic as Na2O2 reacts violently with w



Question Video Determining The Empirical Formula Of Sodium Peroxide Na O Nagwa



What Happens When I Sodium Metal Is Dropped In Water Ii Sodium Metal Is Heated In Free Supply Of Air Iii Sodium Peroxide Dissolves In Water
Sodium peroxide Revision Date 19Jan18 General Advice Immediate medical attention is required Show this safety data sheet to the doctor in attendance Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes Immediate medical attention is required Keep eye wide open while rinsingSynonym(s) Hydrogen peroxideSodium carbonate adduct Formula Na2CO3 15H2O2 Formula weight CAS Number UN Number UN1479 Hazard Class 51 Packing Group II Harmonized Tariff Code 2699 Hazard Statements H272H318 May intensify fire;The molecular or chemical formula of Sodium peroxide is Na 2 O 2 Disodium dioxide is a granular solid which is yellowwhite to yellow in colour When it is mixed with any combustible material it readily ignites by heat, contact with moisture, or by friction When exposed to heat for a longer duration it undergoes vigorous decomposition



Sodium Peroxide



Write The Formula Using Criss Cross Method For Sodium Hydroxide Brainly In
Knowing the mass of hydrogen peroxide contained in the sodium percarbonate, one can calculate the amount of hydrogen peroxide 0468 g H 222 22 22 O 1 mol H O 3402 g H O × = mol H O Recognizing the 23 mole ratio within the sodium percarbonate compound, we calculated the number of moles of sodium carbonate (B) in the originalAlthough sodium peroxide is a thermodynamically stable oxide in air (P(O 2) = 021 atm), sodium oxide usually exists stably due to the reduction effect of nonburned sodium The corrosion reaction occurring in this case is the same as that caused by chemical reaction with the oxygen dissolved in sodium The corrosion is expressed by the formula shown subsequentlyThe negatively charged peroxide ion (O 2 2) is present in inorganic compounds that may be regarded as salts of the very weak acid hydrogen peroxide;



Reactive Chemicals Nature Of The Hazard General Categories


What Is The Name Of Na2o2 Quora
Feb 15, 11 · Sodium carbonate peroxyhydrate (also called sodium percarbonate) is not the same as hydrogen peroxide but when it is dissolved in water, it releases hydrogen peroxide and sodium carbonateFormula Molecular Weight Sodium Peroxide 100 Na 2 O 2 7798 g/mol 4 FIRSTAID MEASURES Eyes In case of eye contact, rinse with plenty of water and seek medical attention Inhalation Move casualty to fresh air and keep atSODIUM PEROXIDE reacts violently with reducing agents, combustible materials and light metals Reacts exothermically and rapidly or even explosively with water to form a strong base (NaOH) and oxygen (O2) Handling Chemicals Safely 1980 p 854


Na2o2 Chemical Safety Models Suppliers Regulation And Patents



Sodium Peroxide An Overview Sciencedirect Topics



Answered Write The Correct Chemical Formula For Bartleby



Sodium Hydroxide Caustic Soda Storage Tanks Naoh Specifications



Sodium Peroxide Youtube



Sodium Hydroxide Wikipedia
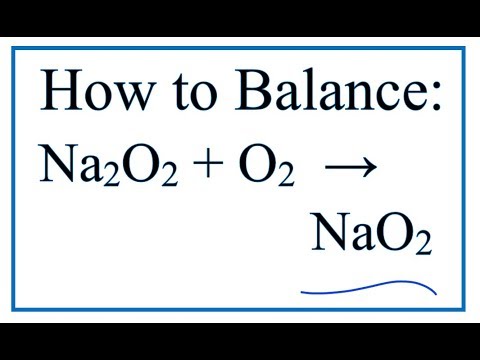


How To Balance Na2o2 O2 Nao2 Sodium Peroxide Oxygen Gas Youtube



Sodium Hypochlorite Bleach Swimming Pools Cleaning Products Compound Interest


Sodium Peroxide Inorganic Compound Formula Na2o2 Stock Illustration



Sodium Peroxide


Sodium Peroxide Suppliers Buyers Distributors Manufacturers In India


Solved Ref Apter Problems Give The Empirical Formula Tha Chegg Com


Sodium Peroxide Na2o2 Pubchem



Fluka Sodium Peroxide For Wurzschmitt Decomposition Acs Reagent Beads Small 95



Solved Sodium Peroxide Is An Oxidizer Used To Bleach Anim Chegg Com



Solved Add 2 Ml Of Sodium Hydroxide To 1 Ml Of Potassium Chegg Com


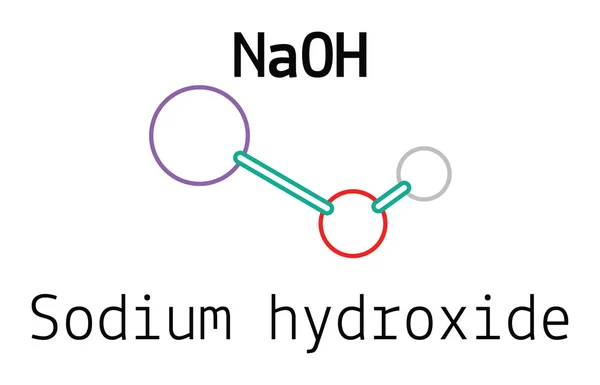
Sodium Hydroxide Naoh Pubchem


Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science



Sodium Hydroxide Lye Learn All About Lye How To Make Soap Safely



Common Names Of Some Chemical Compounds Sodium Carbonate Sodium Hydroxide



Sodium Peroxide At Rs 130 Kg Disodium Peroxide Na2o2 1313 60 6 Disodium Dioxide Sodium Dioxide A B Enterprises Mumbai Id


Sodium Peroxide


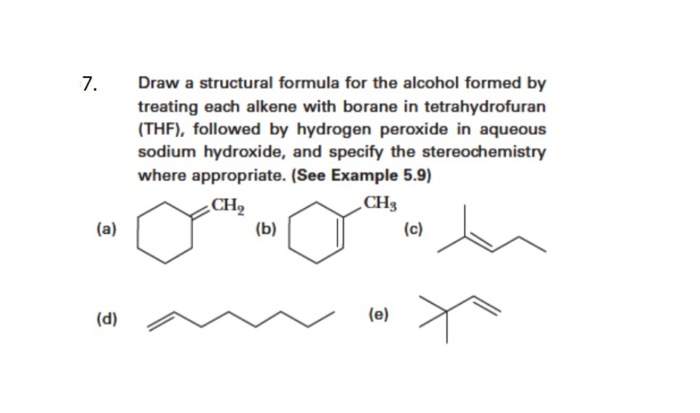
Solved Hydroboration Draw A Structural Formula For The Alcohol Formed By Treating Each Alkene With



Using Sodium Hydroxide Solution To Identify Metal Ions Video Lesson Transcript Study Com



Sodium Peroxide Acs Reagent 93 0 1313 60 6 Sigma Aldrich



What Is Sodium Hydroxide Formula Reactions Video Lesson Transcript Study Com
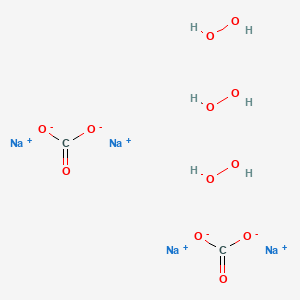


Sodium Percarbonate C2h6na4o12 Pubchem
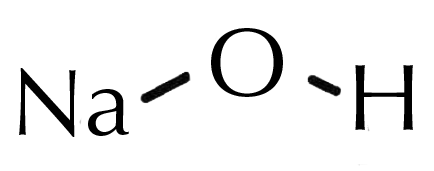


Why Lye How Is Lye Used In Skincare The Dermatology Review



Sodium Peroxide Chemical Formula Inside Orange Stock Vector Royalty Free



Sodium Hydroxide 1310 73 2
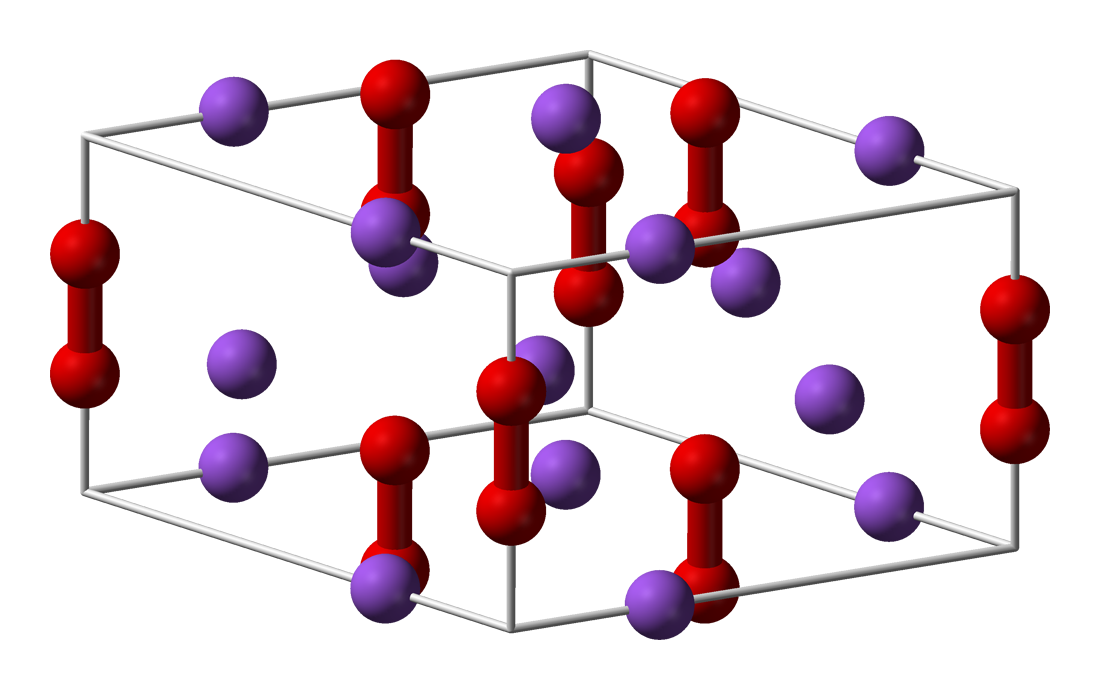


Sodium Peroxide Wikipedia


Sodium Hydroxide Naoh Is Classified As A Strong Base For Every Mole Of Sodium Hydroxide Added To A Large Volume Of Water One Mole Of What Ion Enters The Solution Socratic



Sodium Peroxide An Overview Sciencedirect Topics



What Happens When Zinc Is Put Into Concentrated Sodium Hydroxide Quora
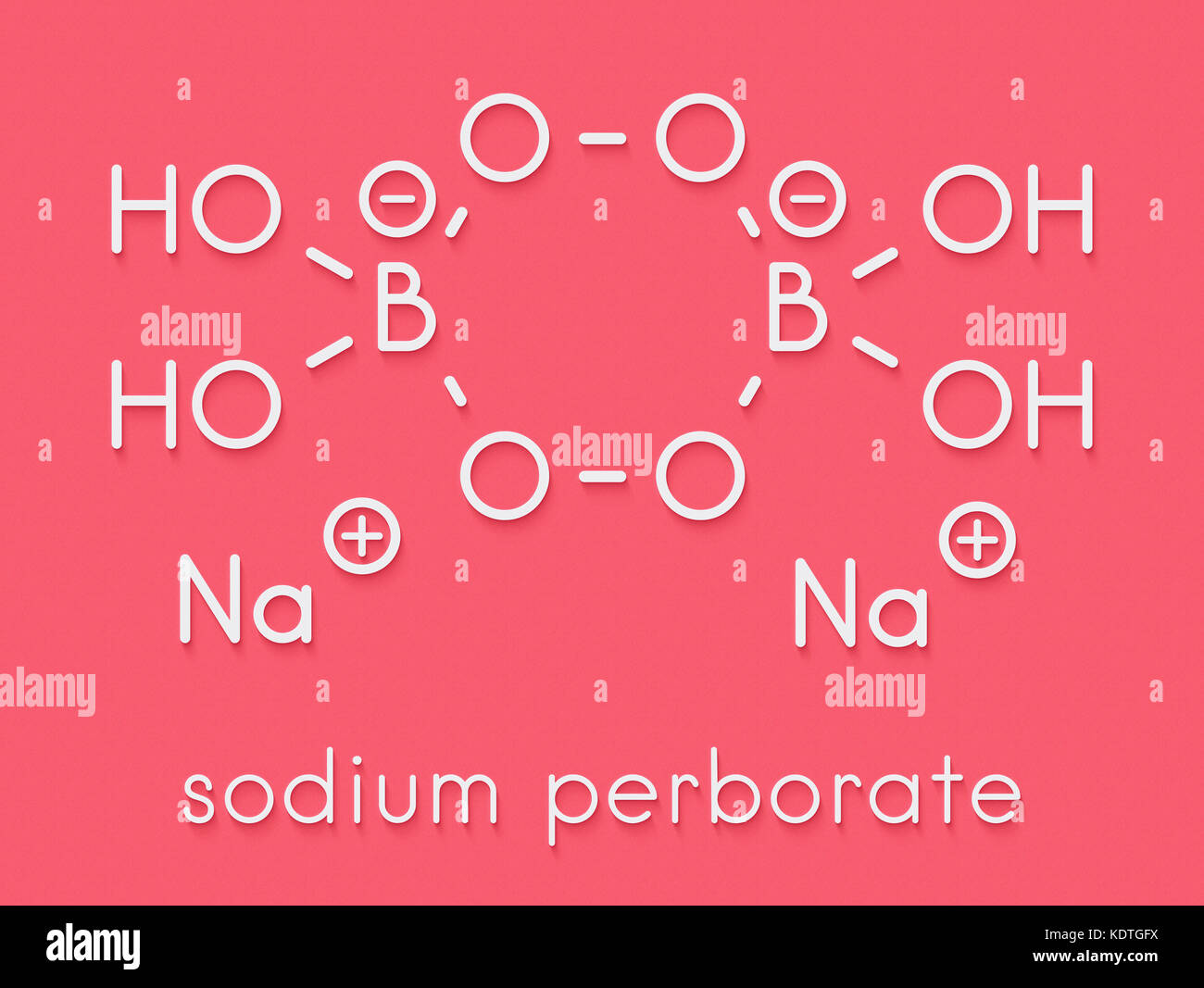


Sodium Peroxide High Resolution Stock Photography And Images Alamy



Sodium Peroxide Wikipedia



Sodium Hydroxide How Is Sodium Hydroxide Made Properties Uses


Sodium Hydroxide Caustic Soda Storage Tanks Naoh Specifications



Lesson Explainer Properties Of Sodium And Its Compounds Nagwa



Sodium Peroxide Science Lab Ltd
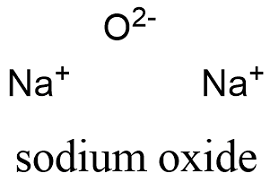


Sodium Oxide Formula



Epb1 Polymeric Diacyl Peroxides Google Patents
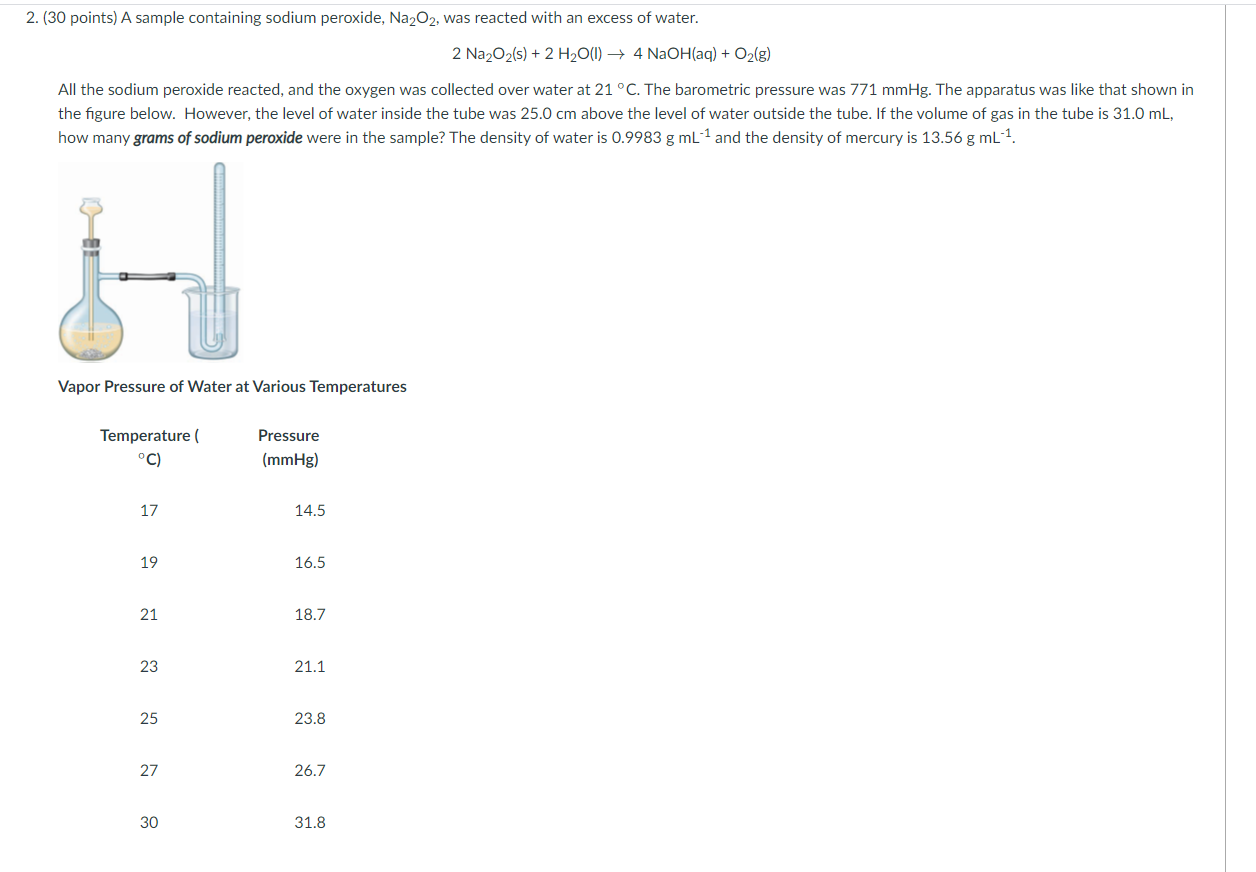


Is Na2o2 A Peroxide
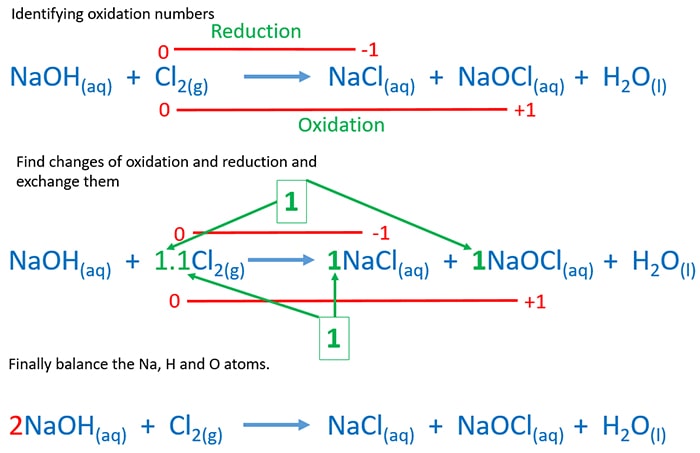


Sodium Hydroxide And Chlorine Gas Reaction Naoh Cl2
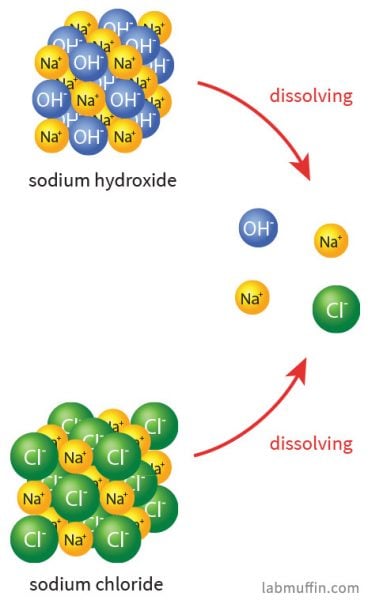


Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science



Sodium Peroxide Youtube



Sodium Peroxide Cas 1313 60 6 Chemical Physical Properties By Chemeo



How To Write The Formula For Naoh Sodium Hydroxide Youtube
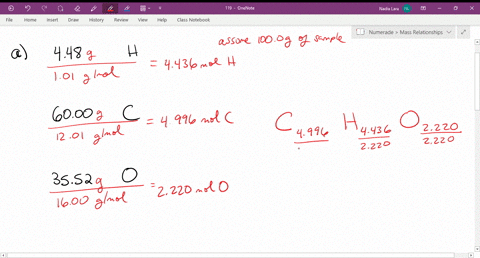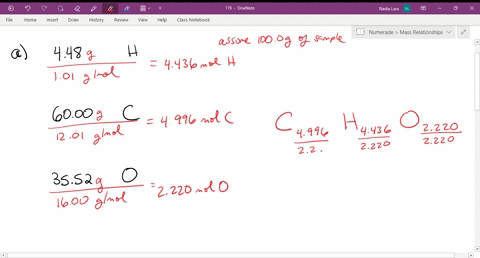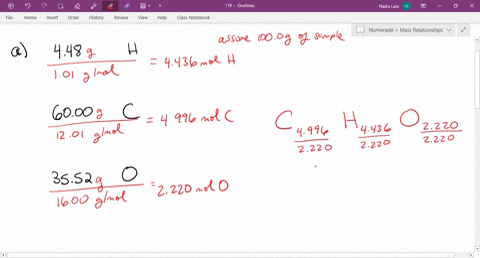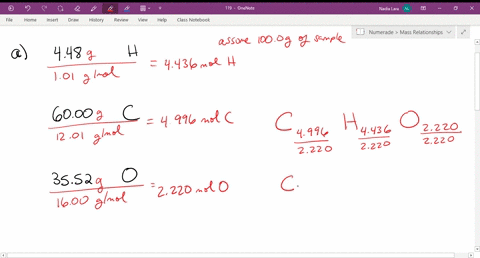


Solved Give The Empirical Formula That Correspond



Sodium Peroxide A Yellow Solid When Exposed To Air Becomes White



How To Balance Na2o2 H2o Naoh O2 Sodium Peroxide Hot Water Youtube
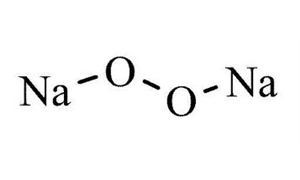


1313 60 6 Cas Sodium Peroxide Granular Peroxides Article No



Sodium Superoxide 978 613 0 963 9
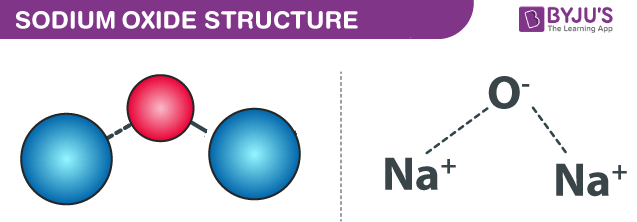


Sodium Oxide Na2o Structure Properties Uses Faqs



Section 6 3 Formulas Of Compounds 1 To Understand The Meaning Of Empirical Formula 2 To Learn To Calculate Empirical Formulas 3 To Learn To Calculate The Ppt Download


2 073 Best Sodium Molecule Images Stock Photos Vectors Adobe Stock



Sodium Peroxide At Thomas Scientific



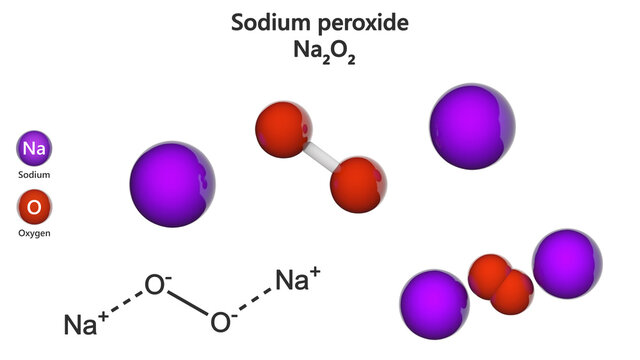
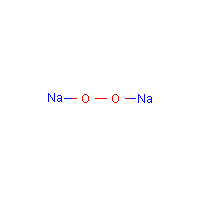
Charge On Sodium Ion Is Plus 1 And Peroxide Is 2 Then The Formula Of Sodium Peroxide Is A Na2o2 B Na2o Chemistry Classification Of Elements And Periodicity In Properties Meritnation Com



How To Find The Oxidation Number For O In Na2o2 Sodium Peroxide Youtube


60 Sodium Hydroxide Vectors Royalty Free Vector Sodium Hydroxide Images Depositphotos


Shutterstock Puzzlepix


Sodium Peroxide Hazardous Agents Haz Map



Which Of The Following Is Formed By The Action Of Water On Sodium
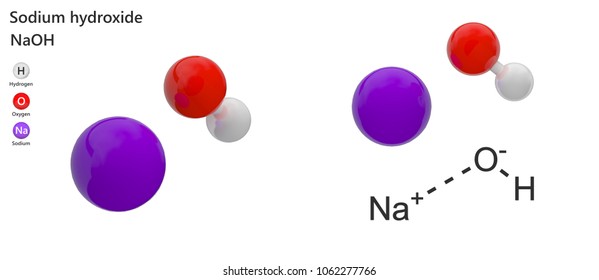


Sodium Hydroxide High Res Stock Images Shutterstock



Sodium Peroxide An Overview Sciencedirect Topics



99 Pure Sodium Percarbonate 2 Lb Bottle Solid Hydrogen Peroxide Sodium Carbonate Hydrogen Peroxide Sodium Carbonate Peroxyhydrate Oxygen Bleach Amazon Ca Health Personal Care
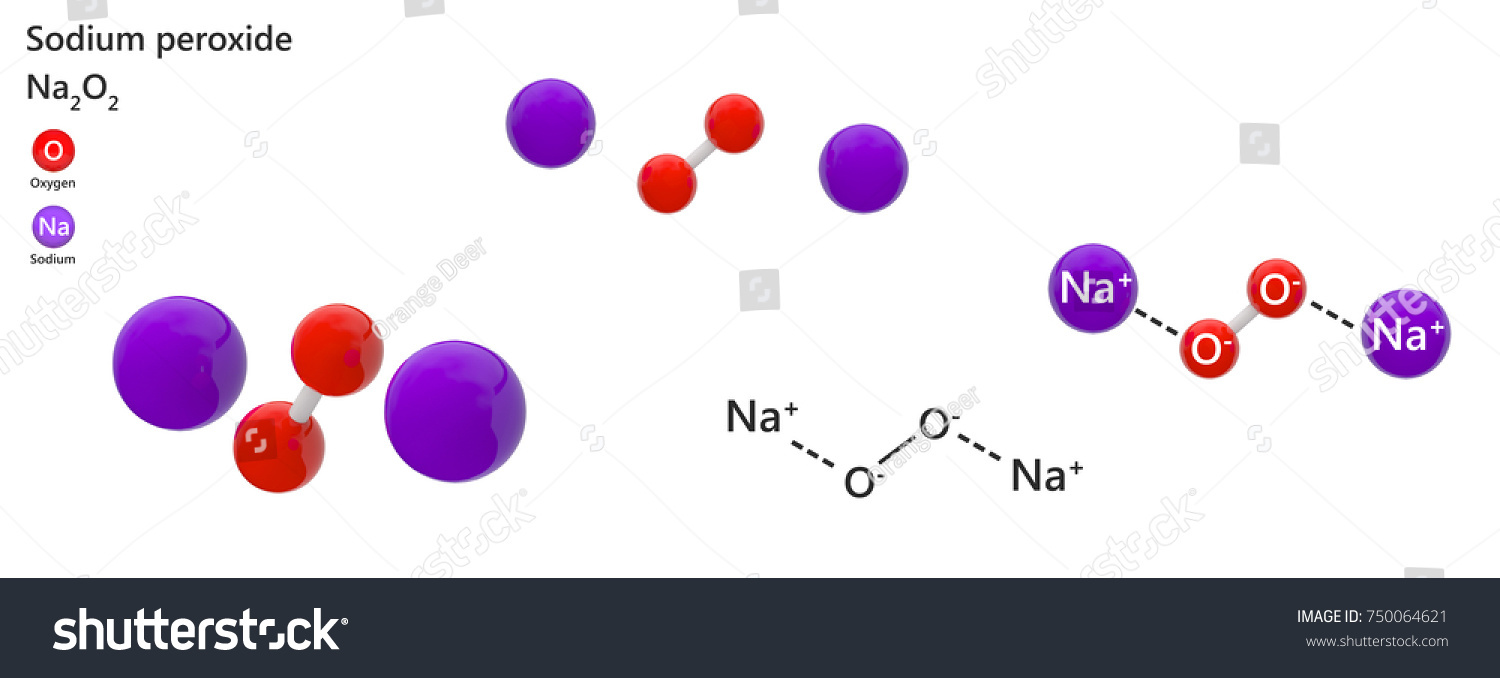


Sodium Peroxide Inorganic Compound Formula Na2o2 Stock Illustration



What Is Sodium Hydroxide Formula Reactions Video Lesson Transcript Study Com
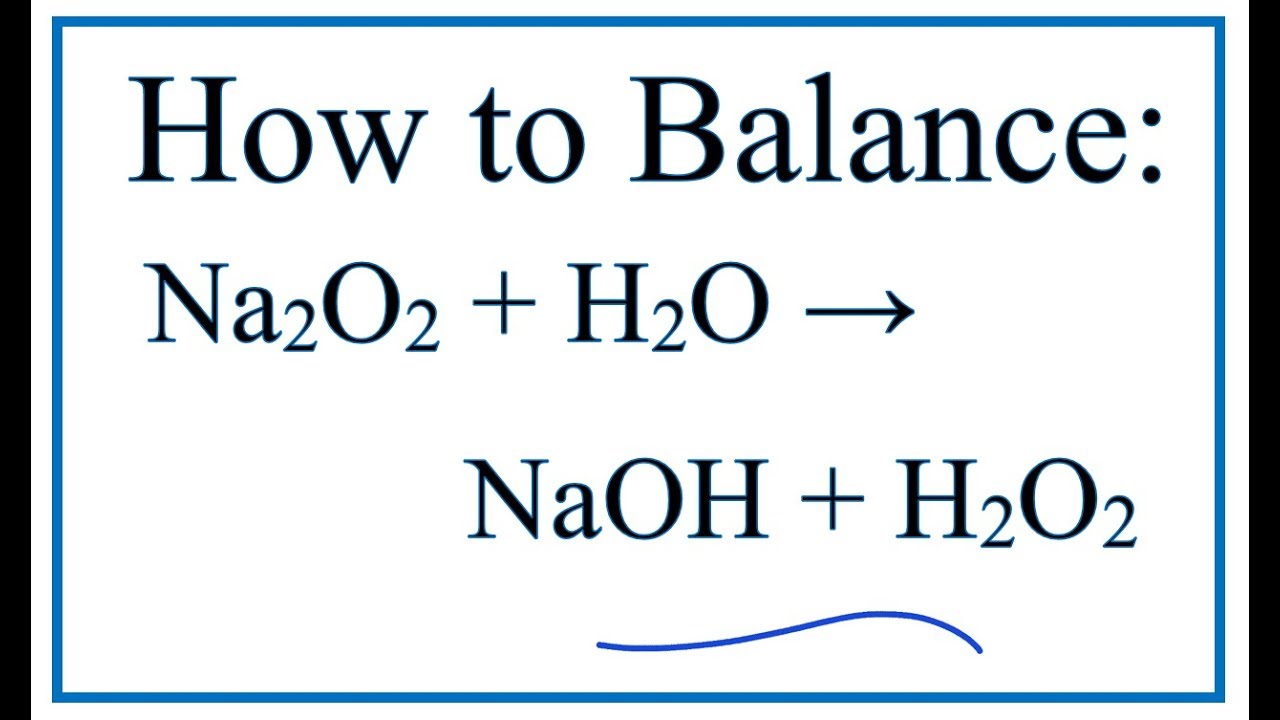


How To Balance Na2o2 H2o Naoh H2o2 Sodium Peroxide Cold Water Youtube


Formula Sodium Peroxide Rx



Hydrogen Peroxide 30 Stabilized With Sodium Stannate Certified Fisher Chemical Fisher Scientific



Sodium Chemical Properties Britannica
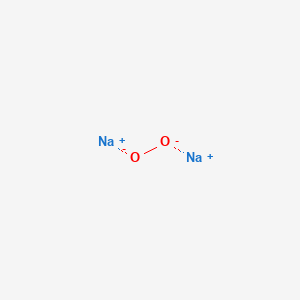


Sodium Peroxide Na2o2 Pubchem


Solved 7 Draw A Structural Formula For The Alcohol Forme Chegg Com



Solved Empirical Formula Problems Give The Empirical Form Chegg Com



Is Sodium Hydroxide Safe In Beauty Products Lab Muffin Beauty Science



0 件のコメント:
コメントを投稿